QUICK TAKES
- A scoping review in Frontiers in Medicine assessed HBOT effects on insulin resistance and related markers.
- Researchers screened 230 studies and included 17 that addressed insulin, insulin sensitivity, or insulin resistance.
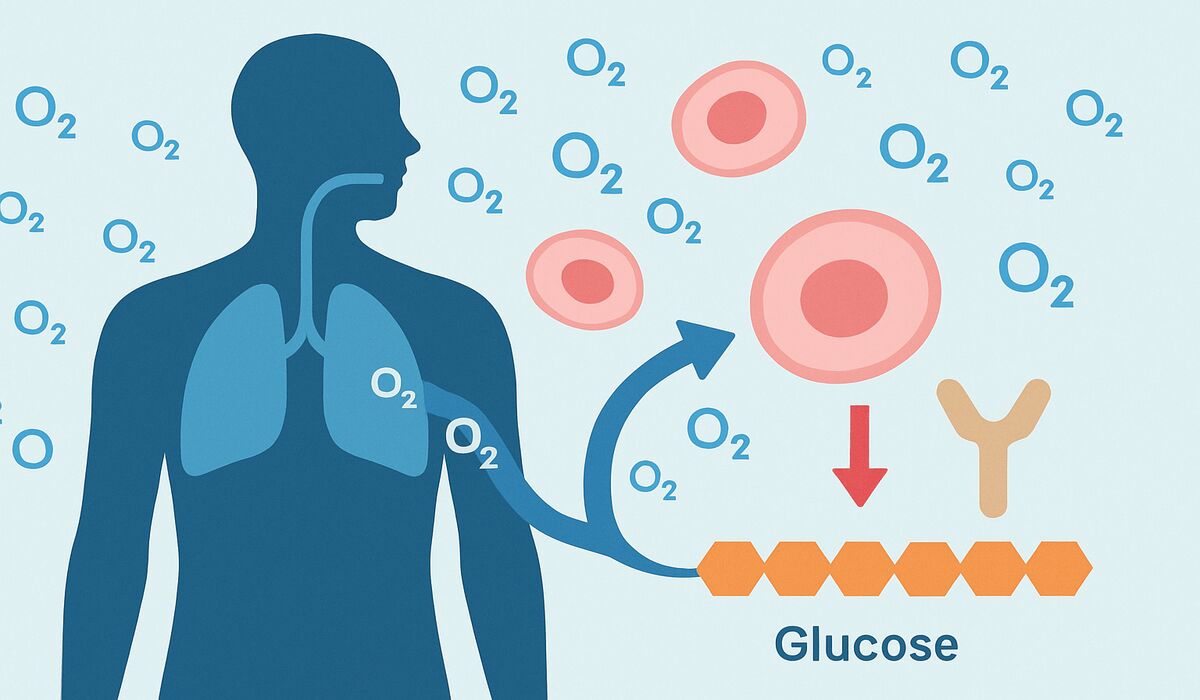
- The review reports HBOT appears to improve fasting glycaemia and decrease insulin resistance, with effects noted after one session.
- HBOT also reduced levels of proinflammatory cytokines that contribute to insulin resistance, but duration of benefit remains unknown.
- Authors state translation into clinical care requires robust randomized clinical trials (RCTs) that are currently lacking.
A scoping review published in Frontiers in Medicine evaluated available evidence on Hyperbaric Oxygen Therapy (HBOT) and its effects on insulin resistance in patients with Diabetes Mellitus (DM).
Authors Mafalda Sampaio Alves et al. screened 230 articles and selected 17 for analysis. The review reports that “the HBOT appears to improve fasting glycaemia and decrease insulin resistance in patients with DM, with effects appearing after 1 treatment session.”
The paper highlights reductions in proinflammatory cytokines but notes the persistence of these effects and the responsible molecular mechanisms remain unclear. (Source: Frontiers in Medicine.)
Hyperbaric Oxygen Therapy (HBOT) is an established treatment modality for specific indications such as diabetic foot ulcers.
The review authors note that formal indications for HBOT do not currently include most diabetes-related comorbidities.
The paper cites the global burden of diabetes and associated mortality; the World Health Organization provides global diabetes data and context on diabetes-related deaths. (Sources: Frontiers in Medicine; World Health Organization.)
Methods and scope
The team used a scoping review methodology to gather all available data addressing insulin, insulin resistance, or insulin sensitivity in the context of HBOT.
Exclusion criteria included studies that did not address insulin resistance, those using normobaric oxygen only, and records without translation into English, Spanish, or Portuguese. From 230 initial records, 17 studies met the eligibility criteria and were analyzed. (Source: Frontiers in Medicine.)
Findings
- Clinical signals: The review reports consistent findings across included studies that HBOT is associated with improved fasting blood glucose and reduced measures of insulin resistance, sometimes after a single treatment session.
- Inflammation: Multiple studies in the review observed reductions in proinflammatory cytokines, biological mediators that can impair insulin signaling and contribute to insulin resistance. Brief explanation: proinflammatory cytokines are signaling proteins (for example, interleukins and tumor necrosis factor) that promote inflammation and can interfere with insulin action.
- Uncertainties: The review notes that the duration of improved insulin sensitivity after HBOT is not established and that the specific molecular drivers of the effect remain undefined. The authors call for higher-quality randomized clinical trials to confirm efficacy and safety. (Source: Frontiers in Medicine.)
Clinical implications and limitations
The authors conclude HBOT shows potential as an intervention to improve glycaemic control and insulin sensitivity in patients with DM, but they stop short of recommending routine clinical use for this purpose pending RCT evidence.
The review does not provide a single standardized HBOT protocol; the studies reviewed used varied treatment regimens, so there is no consensus in the paper on exact pressure, session length, or cumulative exposure required to achieve the reported effects.
For formal HBOT indications and practice standards, the Undersea and Hyperbaric Medical Society (UHMS) maintains guidance and position statements.
Next steps and research priorities
The review identifies several research priorities: well-designed randomized clinical trials to test HBOT against standard care or sham controls for insulin resistance outcomes; standardized reporting of HBOT parameters (pressure in atmospheres absolute, session duration, and number of sessions); and mechanistic studies to map cytokine and molecular pathway changes.
ClinicalTrials.gov lists ongoing and completed trials across HBOT indications and can be searched for trials targeting metabolic endpoints. (Sources: Frontiers in Medicine; ClinicalTrials.gov.)
Sources
- Frontiers in Medicine: scoping review by Mafalda Sampaio Alves et al. (article in Pulmonary Medicine section). (Frontiers in Medicine review)
- World Health Organization: diabetes fact sheet and global mortality data. (World Health Organization diabetes fact sheet)
- Undersea and Hyperbaric Medical Society: HBOT indications and practice resources. (Undersea and Hyperbaric Medical Society)
- ClinicalTrials.gov: registry for randomized clinical trials and study protocols. (ClinicalTrials.gov)
Note on reporting
This article summarizes findings and conclusions presented by the authors of the Frontiers in Medicine scoping review. No additional experimental results or unpublished statements were introduced. Direct phrases from the review are presented in italics and attributed to the original publication.