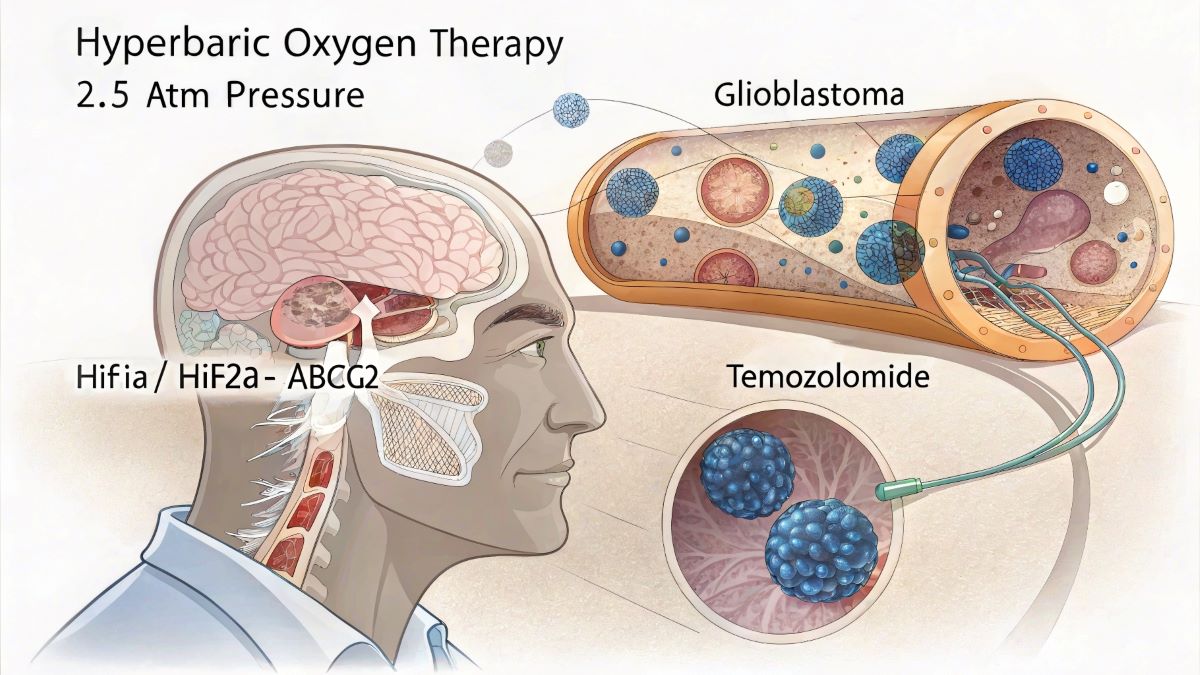
- Researchers from Chongqing University found hyperbaric oxygen therapy (HBO) increases chemosensitivity in glioblastoma cells by disrupting a key resistance pathway.
- The study identifies the molecular mechanism: HBO inhibits HIF1α and HIF2α proteins, which normally promote ABCG2 expression – a drug efflux transporter that causes chemotherapy resistance.
- Surprisingly, HBO treatment alone actually promoted tumor growth and shortened survival time in animal models.
- Combined HBO-temozolomide (TMZ) therapy showed significantly reduced tumor size and prolonged survival compared to TMZ alone.
- The findings, published April 30 in Frontiers in Molecular Neuroscience, suggest HBO could potentially enhance standard glioblastoma treatment.
Researchers have discovered a new mechanism explaining why hyperbaric oxygen therapy (HBO) enhances the effectiveness of chemotherapy in treating glioblastoma, the most aggressive form of brain cancer. The study, published yesterday in Frontiers in Molecular Neuroscience, demonstrates that HBO increases chemosensitivity by inhibiting a molecular pathway involving hypoxia-inducible factors and a key drug resistance protein.
Understanding the Mechanism
The research team from Chongqing University in China identified that HBO works by suppressing the expression of two proteins – HIF1α and HIF2α – which normally help cancer cells survive in low-oxygen environments. These proteins promote the expression of ABCG2, a drug efflux transporter that pumps chemotherapy drugs out of cancer cells, reducing treatment effectiveness.
“This study confirmed that hyperbaric oxygen can inhibit ABCG2 expression through HIF1α and HIF2α, thereby promoting the proliferation and chemosensitization of gliomas,” wrote lead authors Sheng Gong and Pan Wang.
Through a series of laboratory experiments and animal studies, the researchers demonstrated that HBO treatment significantly decreased the expression of HIF1α, HIF2α, and ABCG2 in glioblastoma cells. When HBO was combined with the chemotherapy drug temozolomide (TMZ), there was a marked increase in cancer cell death and reduction in tumor growth.
| Table 1: Effects of HBO Treatment on Glioblastoma With and Without TMZ | |||
|---|---|---|---|
| Treatment Condition | Tumor Growth | Cell Apoptosis | Survival Time |
| Normoxia + DMSO (Control) | |||
| HBO + DMSO |
+117%* |
-35%* |
-11%* |
| Normoxia + TMZ |
-48%* |
+15%* |
+9%* |
| HBO + TMZ |
-94%*† |
+78%*† |
+27%*† |
*p < 0.05 compared with Normoxia + DMSO (Control) Note: For tumor growth, fewer dots indicate better outcomes (smaller tumors). For cell apoptosis and survival time, more dots indicate better outcomes. HBO: hyperbaric oxygen; TMZ: temozolomide. | |||
| Table 2: Protein Expression Levels Under HBO vs. Hypoxic Conditions | |||
|---|---|---|---|
| Protein | Expression in Hypoxic Conditions | Expression with HBO Treatment | Change with HBO |
| HIF1α | ↓ 91% | ||
| HIF2α | ↓ 92% | ||
| ABCG2 | ↓ 84% | ||
| CD133 (stem cell marker) | ↓ 74% | ||
| CD15 (stem cell marker) | ↓ 76% | ||
Note: Circles represent relative expression levels averaged across U87 cells, GBM cells, and tumor tissue samples. Fewer circles with HBO indicate decreased protein expression, which correlates with increased chemosensitivity. HIF: hypoxia-inducible factor; ABCG2: ATP-binding cassette subfamily G member 2 (drug efflux transporter). | |||
Dual Effects Observed
One of the most surprising findings was that HBO treatment alone actually increased tumor growth and shortened survival in experimental models. However, when combined with TMZ, the treatment significantly reduced tumor size and extended survival compared to TMZ alone.
Dr. Nan Wu, the study’s corresponding author, explained that these seemingly contradictory effects stem from the same mechanism. The research showed that while HBO inhibits stemness markers in cancer cells (making them more vulnerable to chemotherapy), it also promotes cell proliferation when used alone.
This dual effect highlights the importance of using HBO only as an adjunct therapy alongside standard chemotherapy treatments for glioblastoma, not as a standalone treatment.
Clinical Implications
Glioblastoma remains one of the deadliest forms of cancer, with most patients surviving only 12-24 months after diagnosis. The findings from this study suggest that adding HBO to standard TMZ treatment could potentially improve outcomes.
The research provides a molecular explanation for previous clinical observations that HBO can enhance chemotherapy efficacy in glioblastoma patients. By identifying the specific pathway involved, the study opens up possibilities for developing new therapeutic approaches that target this mechanism.
“There is a general consensus that HBO therapy combined with temozolomide treatment can increase chemotherapy sensitivity and effectively inhibit the growth of gliomas,” the researchers noted, while emphasizing that more clinical studies are needed to validate these findings.
The study represents an important step forward in understanding how to improve treatments for this devastating form of brain cancer, by revealing the molecular interactions between hyperbaric oxygen, hypoxia-response proteins, and chemotherapy resistance mechanisms.
Citation: Gong S, Wang P, Liao B, Zhao L and Wu N (2025) Hyperbaric oxygen promotes both the proliferation and chemosensitization of glioblastoma cells by inhibiting HIF1α/HIF2α-ABCG2. Front. Mol. Neurosci. 18:1584407. doi: 10.3389/fnmol.2025.1584407